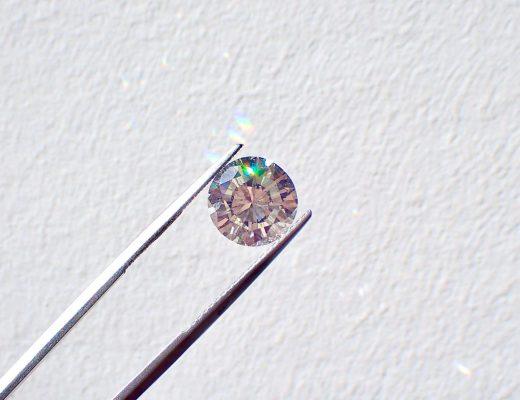
You might have seen the letters CTW stamped on diamond jewelry labels, perhaps on the inside band of a diamond ring or on the clasp of a diamond pendant necklace. Do…
-
-
Your New Year’s Eve Proposal New Year’s Eve is one of the most special days of the year, with flowing champagne, twinkling lights, and an air of celebration all around! It’s…
-
There is no gift that is greater or more meaningful than an engagement ring. Your significant other will proudly wear this piece of jewelry for the rest of her life, so…
-
The tradition of proposing marriage with a diamond engagement ring traces back to the 1400s, when Archduke Maximillian of Austria gave his bride-to-be a sparkling diamond ring. But in today’s world,…
-
There’s nothing more beautiful than a sparkling diamond, especially when it’s used to create a breathtaking piece of jewelry. This beauty often comes at a steep price, though. Why are diamonds…
-
There are many different ways to make a piece of diamond jewelry stand out. Designers can add a pop of color with gemstones or create unique and delicate shapes with filigree.…